GREENHOUSE EFFECT
"A layman’s perspective of the phenomenon of the greenhouse effect"
Electromagnetic (EM) Spectrum (see Figure 1) broadly comprises of Gamma rays, X- rays, UV rays, Visible light, Infrared rays, Microwaves and Radio waves. These bands of the spectrum are identifiable by their differing wavelengths or energies. Wavelength and energy are inversely proportional to each other. Thus, higher wavelength implies lower energy and vice versa. Visible light has a wavelength in the range of 400 to 700 nm. The spectrum having wavelengths shorter than Visible light includes the high energy Gamma rays, X rays and UV rays. Likewise, Infrared rays, Microwaves and Radio waves have longer wavelengths relative to Visible light. In other words their energy is lower compared to the Visible spectrum.
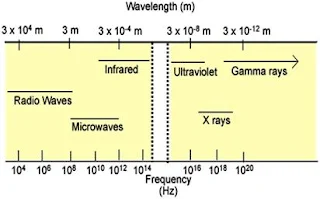 |
| Figure 1. The electromagnetic spectrum |
The essentiality of discussing the EM spectrum in the context of greenhouse gases stems from the fact that the atmospheric gases are permeable to the different components of the spectrum in varying capacities. Primarily composed of Oxygen, Nitrogen, Hydrogen, Carbon dioxide, Water vapour, Nitrous Oxide, Methane, Ozone, etc, the atmosphere readily permits transmission of Visible, Radio frequency ranges and to some extent Microwave and Infrared categories. The shorter energy wavelengths are largely absorbed by the protective Ozone layer. The Ozone layer is located in the second layer of earth’s atmosphere, the stratosphere.
Fortunately for the mankind, the atmosphere provides a sort of greenhouse effect by holding on to the solar energy absorbed by land surface, water bodies, grasslands, ice sheets, desert soil, etc helping sustenance of life. The shorter wavelength energy (primarily Visible and Radio waves) so absorbed is converted into heat and then re-radiated back in the form of longer wavelength infrared radiation. However, it is the ability of the greenhouse gases to absorb this infrared radiation scattered back rather than letting it go which leads to the greenhouse effect. This is due to the fact that atoms of the greenhouse gases, viz, Carbon dioxide, Water vapour, Methane, Nitrous oxide, are loosely bound to each other. Therefore, these atoms begin to vibrate after absorption of the longer wavelength Infrared radiation. Sooner or later, the vibrating molecule of the greenhouse gas emits its additional energy gained from the Infrared radiation. This energy is then reabsorbed by another greenhouse gas molecule. The emission and reuptake results in retention of heat near the surface of the earth, necessary to maintain a perfect temperature. IN ESSENCE THE GREENHOUSE GASES ARE TRANSPARENT TO VISIBLE, RADIO WAVES AND OPAQUE TO INFRARED. A pictorial representation of the greenhouse effect is shown in Figure 2.
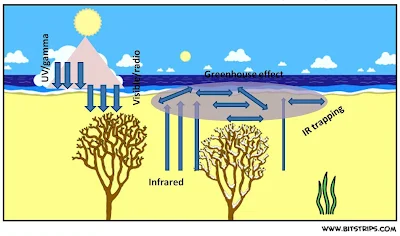 |
Figure 2. Schematic representation of the 'Atmospheric window' responsible for the greenhouse effect ***The End*** Comments on the above article will be greatly appreciated. |







Post a Comment